
Interest in quaternary phosphonium salts began to gain real steam in the middle of the twentieth century, as scientists looked for flexible platforms outside of the more well-known ammonium compounds. Tetrabutylphosphonium chloride didn’t take long to carve out its own niche, thanks to solid researchers in both Europe and North America. In the 1960s, labs seeking alternatives beyond traditional phase transfer catalysts noticed the tough nature and unique solubility profile of this compound, setting it apart from quaternary ammonium cousins. This shift spoke to a bigger theme: curiosity and a willingness to swap out familiar cations in pursuit of new chemical possibilities. Today’s commercial producers have built on this, using more precise manufacturing to scale up purity and yield, but it started with small flasks and some very persistent chemists.
Tetrabutylphosphonium chloride has made its name as an ionic compound with broader uses than many classic phase transfer agents. Its structure—a central phosphorus atom surrounded by four butyl groups and paired with a chloride anion—brings both organic and ionic characteristics into one package. Supply chains for this material favor larger-scale chemical operations, but packaging sizes and grades vary to serve labs, pilot plants, and full industrial processes. You don’t find this in your local store aisle, but chemical catalogs for academic and industrial buyers offer options geared for purity and stability.
This salt most often appears as a white to off-white powder or crystalline chunk, sometimes collecting moisture from air due to its hygroscopic nature. Melting points usually hover in the 80–90°C range, and it dissolves well in polar solvents like water, methanol, and DMSO. The compound’s odor is subtle but can come out more as it breaks down. Tetrabutylphosphonium chloride stands out for its stability: resisting hydrolysis and decomposition even when heated above boiling point of water. That tough attitude has kept it from the shelf-life headaches chemists face with other ionic compounds. On the chemical side, its cationic bulk and lipophilicity help in catalysis and phase transfer—places where ions mingle between water and organic layers or kickstart reactions that rely on balancing charges and keeping actors in the right place.
Most suppliers set tight labeling and technical boundaries for this material. Typical assay levels reach 98% or higher, balancing the needs of analytical chemists and producers working at scale. Water content, as measured by Karl Fischer, usually sits below 0.5%, and manufacturers check for trace impurities like tributylphosphine residues, chloride excess, and iron. Packaging must keep out humidity—polyethylene bottles or steel drums with liners—and each shipment carries safety data sheets tracing regulatory and hazard details under GHS, REACH, or other frameworks. Accurate labeling helps not just for safety reasons, but also for those managing precise stoichiometry in research or plant settings.
Production of tetrabutylphosphonium chloride usually draws on direct alkylation of phosphine by butyl halides. Folks in process plants know this as a two-stage job: start from tributylphosphine and react it with butyl chloride in an inert solvent, often toluene, under carefully controlled temperature. The reaction calls for anhydrous conditions, since any stray water can throw off yield and produce side products. After reaction, the crude salt gets recrystallized and washed, often with nonpolar solvents to strip away leftover organic residues, and then filtered and dried under vacuum. Some researchers use ion exchange or phase transfer conditions to clean up the salt further, especially for electronics applications, where impurities can shorten device lifetime.
Ionic liquids have become a hot trend in green chemistry, and derivatives based on tetrabutylphosphonium chloride lead much of this movement. The core structure lets chemists swap the anion through metathesis—mixing with sodium hexafluorophosphate or other salts to load up different counterions. The parent chloride salt acts as a stable launching pad for further synthesis: you can push it through quaternization reactions, anchor it on solid supports for heterogeneous catalysis, or use it as a ligand or template in metal complex synthesis. The compound’s chemical reactivity ties back to the softness of the phosphonium center and the bulk of the butyl arms, which tweak electron distribution and make new pathways possible compared to classic ammonium systems.
You won’t always see just one name for this material. Chemical catalogs and regulatory registries record synonyms including tetrabutylphosphonium chloride, TBPCl, and phosphonium, tetrabutyl-, chloride. Old publications or international documents translate it as Tetraphenylphosphanium chloride or other spelling variants. In industry, shorthand like “TBPC” crops up, especially in internal documentation or blend specification sheets. Those searching global inventories should confirm CAS numbers as well as trade names because a slip-up in nomenclature can lead to confusion or the wrong product showing up at the dock.
Handling this material means staying aware of both physical and health hazards. The compound carries risk of skin or eye irritation and can affect respiratory health if dust or mist enters the air uncontained. Standard PPE—nitrile gloves, goggles, and lab coats—serves as the front line. Those working around larger volumes often install fume hoods or local exhaust, given the potential for breakdown products under heat. Emergency protocols and spill cleanup kits should be easily at hand, as even minor accidents may lead to persistent odors or surface contamination. Safe storage stays below 30°C, away from acids and oxidizers, and containers must stay tightly closed when not dispensing. Transporters and users comply with regional rules like OSHA, REACH, and country-specific chemical hazard codes—and smart companies keep up to date on shifting standards to avoid compliance surprises.
Tetrabutylphosphonium chloride doesn’t pigeonhole itself into just one industry. In synthesis labs, researchers call on it as a phase transfer catalyst to move anions between layers or kickstart biphasic reactions. The electronics sector leans on it for ionic liquid formulations that cool and lubricate components while allowing high ionic conductivity. Oil and gas companies use it to manage emulsions or as part of demulsifier packages, helping to separate crude into cleaner product streams with less energy loss. Water treatment and green chemistry benefit from its unique solubility and reactivity profile: replacing less stable or more toxic phase transfer reagents in pilot tests and adjusting for process scale-up. In pharmaceutical research, it shows up during the tricky steps where other cationic partners fall short—especially in the context of selectivity or where avoiding nitrogen-based side products matters.
Researchers studying catalysis or green solvent systems keep returning to tetrabutylphosphonium chloride for its versatility. In ionic liquid formulation, this salt delivers wider liquidus ranges and strong thermal stability—critical for designing processes that run hotter or need longer cycles without breakdown. Electrochemical research benefits from the cation structure, as it allows controlled charge transfer and fine-tuning in battery and capacitor prototypes. Scientists try modifying the butyl groups—swapping in linear or branched chains, or adding aromatic components—to push properties in new directions. This trial-and-error culture is fed by both academic curiosity and industrial need: developers hunt for salts that combine low volatility, high conductivity, and environmental safety in applications like carbon capture or chemical separations.
Toxicologists have weighed in on tetrabutylphosphonium chloride, flagging it for moderate toxicity at high concentrations yet not ranking it among the top chemical hazards. Acute exposure can bring respiratory and dermal effects, but chronic exposure studies indicate lower risk compared to many transition metal salts or persistent organic pollutants. Environmental fate studies note that the compound shows less tendency to bioaccumulate, yet limited water solubility means it could persist in soil or water near industrial sites if not handled carefully. Regulatory agencies track environmental release and occupational exposure, and companies tend to err on the side of caution—enforcing closed-system handling, workplace monitoring, and regular health checks for personnel.
Growing concern about safety, green chemistry, and resource efficiency throws new light on compounds like tetrabutylphosphonium chloride. As industries migrate from traditional solvents to ionic liquids, this particular salt carries weight for its tunability and robust performance. Research might soon focus on bio-derived butyl groups or modifications that make downstream degradation less energy-intensive. Circular chemistry concepts—such as recovery and recycling of phosphonium salts or re-integration into new blends—push the envelope. Attention also turns to regulatory adaptation, since shifting global standards could either open up wider use or put pressure on suppliers to redesign formulas for lower environmental impact. As I see it, the compound’s history of adaptability and central role in innovation mean it will stay on the research and process map for years to come, shaping next-generation chemical operations and perhaps leading the charge for safer and more efficient catalysis and solvent technology.
Tetrabutylphosphonium chloride has built a reputation in research spaces and factory labs for its role as a phase-transfer catalyst. In many chemical reactions, water-loving and oil-loving substances refuse to mix. Phosphonium salts bridge this gap, helping reactions move forward efficiently. Over the last decade, I’ve watched scientists quit wrestling with sluggish reactions and start using this salt to coax better yields out of otherwise stubborn processes.
For example, in pharmaceuticals, the push to simplify drug manufacturing sparked demand for reliable catalysts. Tetrabutylphosphonium chloride stands out, especially during the production of complex molecules, such as antiviral and anti-inflammatory compounds. These molecules often need multi-step synthesis, and quick, clean transfer between phases can shave hours—or days—off total production time.
Curiosity about ionic liquids took off about 20 years ago, aiming to replace volatile solvents with greener and safer options. Tetrabutylphosphonium chloride is a base ingredient for making many ionic liquids. These new-age liquids don’t vaporize or burn easily. I’ve seen chemists mix up variations using this phosphonium salt, giving them control over physical properties like viscosity and temperature resistance.
Companies seeking safer processes for extracting rare earth metals or catalyzing polymerization have jumped on this. By swapping out harsh solvents for ionic liquids, accidents in labs and plants have dropped, and waste streams have started to look cleaner. This shift matters, not just for worker safety but also for protecting groundwater and air around chemical plants.
On the environmental front, water treatment remains a growing field. Desalination engineers learned that tetrabutylphosphonium chloride can control scale formation and fouling in plant pipes. Old systems often lost efficiency—sometimes never fully recovering. Now, regular dosing with this salt in pre-treatment keeps pipes clearer and membranes free from stubborn limescale. Fewer shutdowns mean communities can rely on steadier clean water supply, especially in drought-prone regions.
Research has also shown that this compound fights certain biofilms, reducing growth of unwanted bacteria. Freshwater systems are delicate, and keeping microbes down without hammering plants with harsh cleaners is a win, in my view.
Battery developers in Asia and North America started experimenting with tetrabutylphosphonium chloride a few years back. It acts as a heat-stable, chemically resistant additive in electrolytes for lithium-ion and next-gen sodium-ion batteries. Battery makers face a tough challenge balancing performance and safety; one small change in formulation can prevent fires or add years to total cell life.
In electronics manufacturing, certain plating processes use this salt to keep metal ions in solution or to adjust surface qualities of the final product. I’ve heard plant engineers say they see fewer defects and more consistent output since bringing phosphonium salts into the process. With consumer devices getting smaller and more complicated, reliable reagents matter more than ever.
Demand for tetrabutylphosphonium chloride keeps rising as industries look for cleaner, more efficient ways to do chemistry. Tighter safety rules and greater focus on sustainability mean more companies will look for these kinds of ingredients. Researchers can push for safer, cost-effective alternatives in production and wastewater remediation by supporting collaboration among chemical manufacturers, regulators, and end-users. By combining practical lab results with robust oversight and real-world feedback, safer and more efficient chemical processes can become a daily reality outside the labs.
Chemicals shape daily life in more ways than most people see. Tetrabutylphosphonium chloride has developed a specific reputation in research and industry, popping up as a phase-transfer catalyst in chemical synthesis and in some specialty applications. The name rolls off the tongue awkwardly, but the impacts behind it matter for anyone working in a lab or dealing with industrial cleaning products. Too many assume any specialist chemical is either harmless or deadly. The truth usually lives somewhere in the messy in-between.
Safety data for tetrabutylphosphonium chloride comes mainly from lab studies and regulatory filings. Officially, it carries warning labels: skin and eye irritation, respiratory tract discomfort, aquatic toxicity. Accidental splashes can bring on burns or stubborn rashes; breathing in dust leaves your throat tight and lungs irritated. The Material Safety Data Sheet (MSDS) doesn’t leave much to guesswork—“hazardous in case of skin contact, eye contact, or inhalation.” Online forums and research articles back this up, noting that simple exposure mishaps lead to doctor visits more often than people think. The compound’s structure, with phosphonium at its core, means it interacts with cell membranes if it gets inside human tissue, causing cell damage at higher doses.
Still, most folks working with this material take steps to avoid problems: goggles, gloves, proper ventilation, and designated chemical storage. I remember jobs in teaching labs where a sharp reminder from a colleague meant heading for an eyewash station in a hurry after a tiny splash. The discomfort stays with you for hours. Nobody needed convincing after that lesson. You develop habits fast, cleaning up spills the moment they happen, double-checking lids, never leaving gloves off for “just a moment.”
The real danger comes from how easily a chemical causes harm and how likely exposure becomes. Toxicity depends on dose and route of entry. Respiratory or skin contact with tetrabutylphosphonium chloride doesn’t carry the kind of risk you find with hydrofluoric acid or cyanide, but it has clear effects. Long-term effects haven’t been studied as widely, and that leaves gaps in public understanding. A study in the late 2010s pointed to aquatic toxicity, with even low concentrations damaging small fish and disrupting plant growth in water. That got regulators paying closer attention. Nobody wants to see a science experiment turn into an environmental cleanup job.
Acute exposure in people rarely leads to fatalities, but repeated contact invites trouble: dermatitis, aggravated asthma, and eye problems. In an industry context, chronic low-level exposure sometimes slips through, especially if safety rules loosen over time. Larger chemical warehouses or academic labs often handle dozens of such compounds in a day, and small mistakes add up into something bigger.
Risk never disappears, but good routines can make a world of difference. Clear labeling of storage containers, regular staff training, and working fume hoods go a long way. Simple ventilation—opening a window, running a vent fan—reduces the chance of inhaling vapors or dust. Disposal matters, too. Pouring leftover tetrabutylphosphonium chloride down the sink doesn’t just risk local pipes; it sends the chemical into rivers and streams, threatening aquatic life. Following local hazardous waste protocols, sealing containers tightly, and keeping written logs for chemical use all help avoid predictable mistakes.
Many universities and companies now require new staff to complete chemical hygiene training before stepping foot in a real lab. That helps people recognize early symptoms of chemical exposure—a rash, persistent cough, watery eyes—before things get serious. Investing in these basics costs less than dealing with an emergency or a lawsuit. It comes back to taking each compound seriously, recognizing its risks, and building habits that protect both people and the environment.
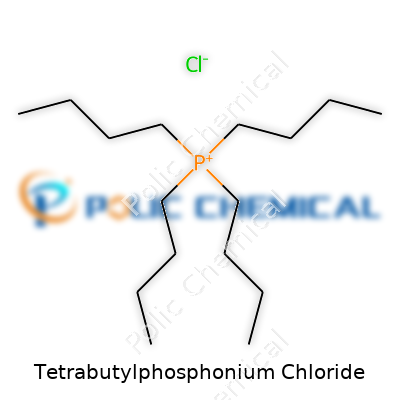
Tetrabutylphosphonium chloride carries the chemical formula C16H36PCl. This shorthand opens up a world of detail about what’s inside each molecule. The structure breaks down into one phosphonium cation—tetrabutylphosphonium—and a chloride anion. Four butyl groups come together around a single phosphorus atom, creating a bulky, positively charged center. Chloride balances this, hanging on through ionic forces.
People who work with chemistry often talk about “quaternary phosphonium” compounds. Tetrabutylphosphonium chloride fits right in. It features a phosphorus atom at the core, with four separate butyl chains (C4H9-) reaching off in different directions. Imagine a star, each arm being a straight-chained hydrocarbon, all tethered to phosphorus. Phosphorus ends up with a formal positive charge since it bonds to four carbons. On the flip side, the chloride ion nestles close by, keeping the ionic charge neutral. You won’t see any covalent bonds between chloride and the rest—just strong electrostatic attraction.
Most folks see a tough string of letters and numbers and tune out. There’s a lot riding on molecular makeup. For me, learning about organic salts like this led to better questions: Why do these molecules act the way they do? The sheer size of tetrabutylphosphonium cations throws roadblocks in the way of forming tight crystal lattices. This has real impact on physical properties—low melting points, high solubility in organic solvents, and resistance to water’s dissolving power. Tetrabutylphosphonium chloride shows up often in research as a phase-transfer catalyst or even in the production of ionic liquids.
One reason the formula matters is safety. Chloride salts carry a reputation, for good reason. The bulky nature of the phosphonium ion makes it less likely to form nerve agents or toxic byproducts compared to smaller organophosphorus chemicals. Still, it’s never wise to overlook safe handling—a lesson learned from many long hours in cold, fume-filled laboratories. Researchers always rely on reliable sources and transparent data sheets. High purity, certification, and traceability directly support safe outcomes in experimental work and industrial processes. This is where Google’s E-E-A-T framework, emphasizing experience and expertise, becomes more than a buzzword. It protects people and the environment.
Scientists face a balancing act using specialty chemicals—effectiveness versus safety. Tetrabutylphosphonium chloride stands out because bulky substituents increase thermal and chemical stability, reducing risks seen with more reactive cousins. Disposal and environmental impact remain top concerns. Breaking down large cations after use strains common wastewater treatment methods. Here, green chemistry enters the conversation. Labs and industrial plants can limit emissions by recycling ionic liquids when possible and switching to lower-toxicity counterions. Training ensures teams understand best practices—no handshake teaching, just thorough, continual learning. Sharing findings across sectors also shrinks the gap between academic discovery and industry application. Every detail, from chemical formula to three-dimensional structure, plays a concrete role in getting chemistry right with real-world consequences.
Anyone who has worked in a lab has probably handled solvents, acids, or powders that quietly demand respect. I remember handling Tetrabutylphosphonium Chloride for the first time. Its oily consistency seemed harmless, but I quickly learned this chemical doesn't play around. Some workers treat chemicals like faceless supplies, but mishandling a salt like this one can go sideways fast. It’s important to remember that this quaternary phosphonium compound not only dissolves in water with ease, but also kicks out heat during the process. If it finds moisture due to loose caps or leaky containers, you can end up with stubborn clumps or worse, exposure risks that creep up long after the lab is closed.
I’ve heard stories of forgotten bottles shoved behind rarely-used reagents, gathering dust. That creates more than clutter—it raises the odds of mishaps. Tetrabutylphosphonium Chloride stands up better to the test of time if stored cool and dry. Humidity does it no favors, so the chemical earns a spot away from sinks, eye wash stations, and room air humidifiers. Keeping it in a tightly sealed container changes the whole game—no spills, no rising moisture. Most suppliers use robust glass or high-density polyethylene bottles. Scraps of cardboard or makeshift lids turn a simple precaution into a roll of the dice. And no chemical should sit on an open bench after work wraps up for the day. I always make space in a chemical-safe cabinet, one with clear labeling, separate from acids and powdered oxidizers that could react if bottles crack or labels fail.
Working with Tetrabutylphosphonium Chloride means using gloves that don’t just shred after one use. Nitrile gloves work well; latex can swell or degrade with some solvents. Safety glasses seem like a pain—until a single splash changes your mind. I’ve learned that even combining small amounts of the chemical with water slowly, under a fume hood, keeps fumes and spills contained. Rushing the job brings trouble: spattered skin, stained gloves, lingering odors that hint at real health risks. For cleanup, absorbents like sand or vermiculite handle accidental spills. Mop up with care and keep waste in a labeled hazardous bin, waiting on proper disposal. Paper towels tossed in regular trash cans spark headaches down the line.
Ignoring the little things can add up: minor burns, headaches from fumes, or skin irritation that lingers through a weekend. The European Chemicals Agency has flagged phosphonium salts like this one for causing skin and eye irritation, especially in higher concentrations. Without regular training and clear protocols, everyone in the lab gets left exposed. Following standardized safety data sheets, holding monthly reviews, and setting up quick-access spill kits create a buffer against that risk. On a busy day, it’s easy to skip steps. But safety routines, like double-checking labels, running a glove check, or storing reagents where nothing can tip or leak, save a lot of grief.
Staying safe never arrives at “done.” Regular audits of chemical stocks cut down on half-used bottles and mystery spills. Electronic records, tied to every batch and storage location, support traceability, making it easier to respond if things slip. Building a strong reporting culture turns every minor incident into a chance for team learning. In my experience, colleagues who feel supported by smart protocols protect not just themselves, but everyone who shares the space.
Chemical processes often demand creative solutions. Among the quirky players, tetrabutylphosphonium chloride grabs some attention. Chemists eye this compound both for its potential as an ionic liquid and for its street-smarts in phase transfer catalysis—a double life worth unpacking.
Ionic liquids sound trendy, but their appeal runs deeper than just catchy names. Tetrabutylphosphonium chloride fits this category because it forms liquids at relatively low temperatures, staying put when air hits it. No flashy smells, not a drop flies off. That stability matters in my own experience with moisture-sensitive reactions. Conventional solvents often pop open, evaporate, or cause headaches with toxicity. This compound resists those problems, and research puts its thermal stability on par with other phosphonium salts.
The structure makes all the difference. The big tetrabutylphosphonium ion disrupts the order inside the liquid, keeping the melting point low. Most chemists reach for imidazolium or pyridinium salts out of habit, but phosphonium choices like this one offer a break from the crowd—especially when it comes to handling strong bases or high temperatures. Diverging from convention often means fewer pitfalls in the reaction flask.
Phase transfer catalysis follows a simple idea: get reactants from different worlds talking to each other. Water and oil won't mix, but add the right helping hand and you spark movement between layers. Tetrabutylphosphonium chloride steps in as that middleman. It carries ions from the watery side to the oily side, making reactions work that would otherwise stall out.
In real labs, I found that switching from old standards like tetraalkylammonium salts to this phosphonium option protected fragile reactants. Chloride ions move cleanly, with less fuss from impurities. Diverse industries—like pharmaceuticals and organic dyes—lean on phase transfer catalysts to avoid tough conditions or wasteful solvents. Literature shows clear evidence for faster, cleaner conversions. This plays out in simple setups just as well as advanced process chemistry.
No chemical is perfect, and due diligence matters. While tetrabutylphosphonium chloride brings non-volatile perks, questions about environmental persistence and end-of-life disposal keep returning in academic and industrial circles. Toxicology isn’t fully mapped. Some labs reported bioaccumulation concerns. The price tag also sidesteps quick adoption for modestly scaled projects, as alternative catalysts might get the same job done for less money.
It helps to remember the direction research is moving. Greener chemistry pushes for less waste and safer chemicals. This compound isn’t a magic ticket, but it checks boxes for solvent reduction and milder reaction conditions. Engineers can plug it into closed-loop systems, recycle it, and recover most of it, which shaves off some downstream impacts. Still, putting more effort into lifecycle analysis and toxicity profiling will future-proof its use.
Plenty of small startups and university labs now work on hybrid ionic liquids and new phase transfer catalysts, searching for the sweet spot between performance and environmental safety. Tetrabutylphosphonium chloride joins that search as both a workhorse and as a reminder: chemistry keeps evolving because folks look past the obvious. Balancing lab practicality, environmental impact, and scalability will shape its role as researchers keep pushing boundaries. Fact-based choices and sharing both triumphs and mistakes will drive the field forward.

| Names | |
| Preferred IUPAC name | tetrabutyl(1λ⁵)-phosphanium chloride |
| Other names |
Tetrabutylphosphanium chloride
Tetrabutylphosphane chloride TBPCl |
| Pronunciation | /ˌtɛ.trə.bjuːˌtaɪl.fɒsˈfəʊ.ni.əm ˈklɔː.raɪd/ |
| Identifiers | |
| CAS Number | 877-91-8 |
| Beilstein Reference | 1519266 |
| ChEBI | CHEBI:63912 |
| ChEMBL | CHEMBL4298607 |
| ChemSpider | 117650 |
| DrugBank | DB11196 |
| ECHA InfoCard | ECHA InfoCard: 100.159.120 |
| EC Number | 215-809-6 |
| Gmelin Reference | 82232 |
| KEGG | C21102 |
| MeSH | D017089 |
| PubChem CID | 18682377 |
| RTECS number | TG3150000 |
| UNII | 9W5V7SFB2U |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | DJ6910000 |
| Properties | |
| Chemical formula | C16H36ClP |
| Molar mass | 339.00 g/mol |
| Appearance | White to off-white solid |
| Odor | Odorless |
| Density | 0.89 g/mL at 25 °C |
| Solubility in water | Soluble in water |
| log P | 1.42 |
| Vapor pressure | 0.00005 mmHg (25 °C) |
| Acidity (pKa) | Acidity (pKa): 12.5 |
| Basicity (pKb) | 8.3 |
| Magnetic susceptibility (χ) | -77.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.487 |
| Viscosity | 542 cP (25 °C) |
| Dipole moment | 8.80 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 322.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | Std enthalpy of combustion (ΔcH⦵298) of Tetrabutylphosphonium Chloride: **-6281.7 kJ/mol** |
| Pharmacology | |
| ATC code | 'Tetrabutylphosphonium Chloride' does not have an ATC code. |
| Hazards | |
| Main hazards | Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| Precautionary statements | P260, P262, P264, P280, P301+P312, P303+P361+P353, P305+P351+P338, P330, P337+P313, P363, P405, P501 |
| NFPA 704 (fire diamond) | 2-0-1 |
| Flash point | > 185 °C |
| Autoignition temperature | Autoignition temperature: 400 °C |
| Lethal dose or concentration | LD50 Oral Rat 750 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 750 mg/kg |
| NIOSH | ST2725000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | Not established |
| IDLH (Immediate danger) | NIOSH has not established an IDLH for Tetrabutylphosphonium Chloride. |
| Related compounds | |
| Related compounds |
Tetrabutylammonium chloride
Tetrabutylphosphonium bromide Tetrabutylphosphonium hydroxide Tetrabutylphosphonium fluoride Tetraphenylphosphonium chloride Tetrabutylammonium bromide |