
Chemists first managed to prepare trihexylphosphine in lab experiments during the explosive growth of organophosphorus chemistry in the twentieth century. Trade and industrial demand for advanced ligands, beginning after World War II, drove research teams to expand the boundaries of phosphine ligands beyond simple phenyl or methyl derivatives. Laboratories in Europe and North America, responding to rising needs in catalysis and extractive metallurgy, found long-chain trialkylphosphines like trihexylphosphine an attractive avenue to tinker with solubility and electronic properties. From bench-scale synthesis in university settings, trihexylphosphine gradually found its way onto catalogues of specialty chemical suppliers, offering a new handle for research projects and industrial applications. This development benefitted from the parallel explosion in analytical techniques, which made handling and confirmation of air- and moisture-sensitive organophosphorus compounds much less of a white-knuckle experience.
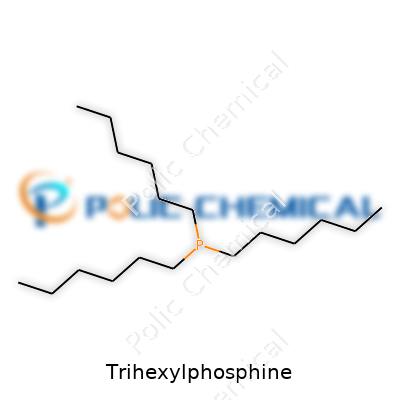
Trihexylphosphine, sporting three straight six-carbon chains on a phosphorus backbone, lands squarely in the long-chain trialkylphosphine camp. Its molecular formula, C18H39P, sets it apart from its shorter cousins like tributylphosphine. Dull mention of the structure easily overlooks how it combines bulk and flexibility, with each hexyl group offering more hydrophobic character and less volatility than common alternatives. Dealers list trihexylphosphine either as a pure liquid or stabilized solution depending on its intended end use, with packaging tightly controlled to minimize contact with air and water, since any exposure leads to rapid oxidation and evolution of unpleasant odors. Suppliers often slap on a colorless-to-pale yellow hue in the product description, which mostly reflects careful purification, as trace impurities easily mar the appearance.
This molecule presents as an oily liquid at room temperature, with a melting point well below ambient, and a boiling point perched high enough to ensure stability during most synthetic operations. Its density hovers in the 0.85 to 0.87 g/cm³ range, lighter than water, and it sports a refractive index notable enough for analysts to use as a diagnostic. It is practically insoluble in water, a trait shared by most long-chain phosphines, while dissolving readily in common organic solvents like toluene, hexane, and dichloromethane. Chemical reactivity centers on the phosphorus atom, which can readily undergo oxidation to yield the corresponding phosphine oxide, and also forms strong complexes with transition metal ions. Its electron-donating ability, measured in Tolman cone angle and electronic parameters, exceeds that of less-sterically encumbered trialkylphosphines, making it valuable in select catalytic cycles. The compound’s unpleasant, garlic-like odor is impossible to ignore in close quarters and betrays its volatile nature even at minor headspace levels. The potential for spontaneous ignition in air, combined with unpleasant fumes, shapes its handling protocols in both academic and industrial settings.
Manufacturers list specifications that address both purity and trace impurities, since most users require trihexylphosphine free from oxidized byproducts, chloride residues, or other phosphorus-containing compounds. Typical product comes with a guarantee of >97% purity, along with spectroscopic documentation to confirm the absence of trihexylphosphine oxide or residual solvents. Labeling rules, shaped by regional regulations, highlight the chemical’s CAS number (referenced in databases for regulatory or supply chain tracking), GHS hazard statements, and pictograms indicating flammability and toxicity. Packaging usually arrives under inert gas, either in glass ampules or PTFE-sealed bottles, with lot-specific printouts to enable traceability. Transport and storage guidelines rest on minimizing vibration, temperature swings, and any risk of breach, since a single compromised bottle can turn an entire workspace into a no-go zone due to its stubborn stench and reactivity.
The classic approach to synthesizing trihexylphosphine builds from phosphorus trichloride. Technicians introduce hexylmagnesium bromide, a Grignard reagent, to PCl₃ under controlled, anhydrous conditions. Reaction setup demands a dry, inert atmosphere since both phosphorus trichloride and the Grignard reagent react violently with moisture. The progress of the reaction, monitored by NMR or thin-layer chromatography, reveals the sequential substitution of chlorine atoms for hexyl groups, with magnesium salts precipitating out as a byproduct. Workup requires careful extraction and distillation under reduced pressure, since trihexylphosphine boils well above 100°C but decomposes when overheated or exposed to air. The final product is typically vacuum distilled and stored under argon to preserve freshness. Each batch may undergo acid-base scrubbing, or degassing, to ensure product meets demanding purity standards for sensitive metal-catalyzed reactions.
Trihexylphosphine’s chemistry mostly plays out in two arenas: oxidation of the phosphorus center and coordination to transition metals. In the laboratory, exposure to oxidants like hydrogen peroxide or even atmospheric oxygen rapidly yields trihexylphosphine oxide—a substance with entirely different physical and chemical properties, favored in solvent extraction processes. Alkylation or arylation reactions are rare due to steric hindrance, but the compound finds its niche as a ligand in homogeneous catalysis. Chemists rely on its electron-rich phosphorus atom to stabilize various states of palladium, platinum, rhodium, and ruthenium, which are essential in fine chemicals manufacturing. Modifications to the hexyl chains themselves are uncommon; more often, researchers focus on blending trihexylphosphine with other ligands to tune catalytic activity. In electrochemical applications, phosphines like this can act as reductants or transfer agents, depending on the potential applied and the supporting electrolyte. The compound rarely participates in direct nucleophilic substitution but does add reactivity to multimetallic complexes in polymer growth or hydrogenation cycles.
Trihexylphosphine also appears under aliases such as phosphorustrihexyl, P(hexyl)₃, or sometimes just THC in laboratory shorthand. Distributors use the standard IUPAC name but may highlight proprietary labels or catalogue codes for internal tracking. Its synonym list includes trialkylphosphine-hexyl and hexylphosphine, though such naming risks confusion with mono- or dihexyl species. On commercial supply sheets, expect to see CAS registry information paired with branding from specialty chemical vendors, especially those catering to the pharmaceutical industry.
Lab veterans know that trihexylphosphine deserves extra respect at the bench. Acute exposure leads to irritant effects in skin, eyes, or respiratory system, compounded by a lingering odor that takes days to leave even a well-ventilated workspace. Spillage, even minor, means immediate escalation to containment procedures. Facilities install chemical fume hoods and equip users with nitrile gloves, goggles, and lab coats because the compound permeates latex and clings to surfaces. Automated systems handle bulk packaging to prevent occupational exposure, while fire suppression relies more on sand or smothering agents since water only spreads a fire caused by phosphines. National and international rules mandate training for anyone handling or transporting this substance, including routine drills on spill response and medical surveillance for those routinely exposed. In case of accidental contact, standard protocol includes removal from exposure, thorough washing, and onsite emergency evaluation, given the potential for chemical burns or sensitization.
Industry and researchers assign trihexylphosphine to roles that exploit its strong sigma-donating ability and bulk. In homogeneous catalysis, an area dominated by transition metal complexes, it fine-tunes selectivity and efficiency in hydrogenation, hydroformylation, and carbonylation processes. Synthetic chemists turn to it for crafting specialty ligands in pharmaceutical development, using its hydrophobic nature to steer reactivity away from unwanted side reactions. Metal extraction specialists in hydrometallurgy favor its oxides for solvent extraction, particularly for isolating rare earth elements from mixed ore streams. Efforts in electronics manufacturing focus on using phosphine derivatives as dopants or modifiers in the synthesis of specialty semiconductors. In academic labs, trihexylphosphine aids fundamental studies in ligand field theory, and its extreme sensitivity sometimes makes it a model compound to probe new purification or analytical techniques.
Ongoing work seeks to adapt trihexylphosphine to cleaner, safer, and higher-yielding chemical transformations. Research teams study its influence on catalyst activity, branching into greener methods for synthesizing fine chemicals or chiral drugs. A big push looks at immobilizing phosphine ligands on solid supports, hoping to combine the selectivity of homogeneous catalysis with easier separation and recycling. Environmental scientists, noticing the persistent odor and risk profile, explore milder oxidants or encapsulation technologies to reduce accidental emissions. Projects that link phosphorus ligands to nanomaterial synthesis show promise, especially where precise tuning of electronic properties is essential for device miniaturization. In medicinal chemistry, the challenge lies in reducing toxicity, with researchers designing trihexylphosphine complexes that deliver new pharmacological activity or catalyze selective bond construction inside biological systems. Thoughtful collaboration between commercial producers and academic inventors plays a key role in moving beyond the bench, scaling up new methods to address process safety, waste minimization, and regulatory compliance.
Toxicologists flag trialkylphosphines as acute hazards, especially in unventilated labs or during accidents. Inhalation provokes respiratory distress, while skin contact produces lasting irritation, both confirmed in case reports and limited animal studies. Chronic exposure links to nervous system symptoms, and the risk of phosphine gas evolution in contact with strong acids highlights the need for constant situational awareness. Researchers rely on LC50 and acute dose metrics derived from rodent tests, which drive occupational limits and spill response plans. Environmental fate studies find that trihexylphosphine, like its shorter analogs, persists in soils and sediments, where it slows degradation and risks bioaccumulation. Disposal practices favor incineration at high temperature, paired with neutralizing scrubs to minimize airborne emissions. Ongoing studies aim to clarify breakdown pathways, hoping to identify safer substitutes or remediation tools in the event of industrial spills.
Looking ahead, the role of trihexylphosphine will likely expand as industries transition toward more specialized, high-value materials. The shift to sustainable processes demands ligands that can operate under milder, less polluting conditions, and trihexylphosphine’s bulk and donation strength make it a strong candidate for customizing next-generation catalysts. Artificial intelligence and automation in reaction monitoring continue to reduce human error, potentially cutting down on accidental releases. Commercial interest rests on finding greener synthetic routes for both the phosphine itself and the products it helps make, with bio-based feedstocks and waste recycling drawing increasing attention. Policy shifts, especially in Europe and North America, stress the importance of safe handling and end-of-life management, prompting companies to rethink packaging, labeling, and even molecule redesign. As part of a broader toolkit for green chemistry, trihexylphosphine offers both challenge and opportunity, matching the industry-wide need for performance with the public’s growing concern over health and environmental safety.
Trihexylphosphine might not roll off the tongue like table salt or aspirin, but for anyone who has tinkered in a chemistry lab or kept up with advances in industrial chemistry, this compound crops up in some fascinating places. It’s a colorless liquid with a telltale smell—if you’ve ever caught a whiff in a lab, you won't forget it. Like many organophosphorus compounds, it carries a certain edge, offering more than just a spot in a warehouse. Trihexylphosphine is a ligand; in other words, it forms partnerships with metals to kick off reactions most people rarely think about but rely on every day.
Chemists often rely on trihexylphosphine for its unique set of skills. In the world of catalysis, especially in processes that drive petroleum, pharmaceuticals, and synthetic polymers, this compound finds its place at the table. Its three hexyl arms give it just enough bulk and flexibility to work as a stabilizer. It binds tightly to metals like rhodium, palladium, platinum, and nickel. Why does this matter? These partnerships help run reactions that turn raw chemicals into familiar products: medicines, plastics, fuels, even flavors and fragrances. Without ligands like trihexylphosphine, many modern conveniences would be a lot harder to produce—or simply not feasible at all.
My own experience with lab work showed the value of the right ligand in a tricky synthesis. A friend working with new catalysts for cross-coupling reactions kept running into trouble with selectivity; contaminants would show up uninvited, even after hours of careful work. Swapping in trihexylphosphine changed the game, allowing for a cleaner reaction that needed less energy and saved precious time. That’s not an isolated story—across research journals and industrial projects, chemists reach for trihexylphosphine because the data backs up its effectiveness.
This compound doesn’t stop at petrochemicals or plastics. Many pharmaceutical breakthroughs trace their roots to faster, more reliable reactions made possible by organophosphine ligands. By fine-tuning catalysts, researchers can build molecules with precision—necessary for medications that target specific proteins. Trihexylphosphine provides a balance of bulk and flexibility that opens new creative avenues. Real-world impact comes in the form of drugs produced at larger scale, with fewer unwanted byproducts. This means cleaner medicines, manufactured in a way that’s less wasteful and more cost-effective.
Chemistry is rarely all upside. Trihexylphosphine demands careful handling; exposure or spills can prove hazardous, and its long-term environmental effects skip easy classification. Chemists need to wear gloves, lab coats, and work in proper ventilation when dealing with any organophosphine compound. Disposal remains tricky: traditional waste streams don’t always cut it, which pushes teams to develop specialized treatment processes. This brings its own costs and complications. The industry pays attention to these risks, funding research into safer replacements or better control systems. Universities and startup labs now focus on greener, less hazardous ligand options, looking to replace compounds like trihexylphosphine without giving up performance.
Hands-on experience in a research group taught me that no substitute appears overnight, but each round of experiments tightens safety standards and pushes innovation forward. Clear guidelines and smart engineering can manage most of the risks for now. The real progress will come from breakthroughs that give chemists similar power but with less downside. Until then, trihexylphosphine remains a mainstay for real-world synthesis and catalysis.
Trihexylphosphine gets attention for its use in serious lab work and high-end chemical synthesis. Its chemical formula stands out: P(C6H13)3 or more simply, C18H39P. That translates to one phosphorus atom and three hexyl chains hanging off of it. Breaking down each hexyl chain, you have six carbons and thirteen hydrogens, multiplied by three, and stuck to phosphorus like the legs of a tripod. You find this stuff in both academic research and commercial production, usually as a clear, oily liquid with a distinctly pungent odor.
Phosphine ligands matter in catalysis and organic chemistry. Trihexylphosphine leads a pack of alkylphosphines valued for how they can fine-tune a metal’s electronic and steric environment. I’ve seen colleagues frustrated by reactions that stubbornly refuse to finish cleanly, only to flip that outcome by swapping out a phosphine. In this field, tiny tweaks in molecular shape or electron richness can mean big changes in yield or selectivity. Trihexylphosphine stands out for its bulk and hydrophobicity, not just the formula. The world’s not short of phosphines, but ones with three unbranched hexyl groups offer a weird combination of size and surface slip. It comes in handy with certain palladium and rhodium catalysts, especially in hydroformylation or when a reaction hates moisture.
This isn’t a backyard chemical. Trihexylphosphine comes with strong warnings. Touching it unprotected can lead to nasty burns; inhaling its fumes isn’t much better, with possible lung or nervous system reactions. It’s not just the elemental phosphorus that demands respect. Many organophosphines react fast with air or oxidize to less-than-pleasant byproducts. From firsthand lab work, gloves and careful ventilation are standard. I remember the relief of switching from glass syringes—always a risk with viscous, sticky liquids—to safer, modern dispensers. That may sound minor, but even experienced chemists prefer tools that cut down handling errors.
Labs produce organophosphorus waste that needs careful disposal. Improper handling contaminates soil and water. Attempts to minimize runoff start with limiting the use of such reagents, but complete avoidance often isn’t realistic in pharmaceutical work or advanced materials science. Researchers work with local authorities and waste specialists to destroy hazardous organics safely—often by incineration at high temperatures in controlled environments. In my experience, people grow more conscious of the downstream effects of every molecule introduced into the ecosystem. By tracking inventories closely and embracing green chemistry approaches, teams make a real difference over time.
Trihexylphosphine isn’t just a formula. It’s a reminder of chemistry’s push-pull between progress and responsibility. For those in labs, remembering both its promise and its dangers is part of daily life. If the next generation of phosphines brings safer properties or cleaner byproducts, credit will go to teams who never stopped weighing the formulas on more than just a molecular scale.
If you’ve spent time in a research lab, chances are you’ve run into chemicals that act like troublemakers. Trihexylphosphine sits squarely in that group. This compound looks harmless in a bottle, but its sensitivity to air and moisture means the wrong storage choice can spark more than just inconvenience. A chemist once told me a tale about a rookie slipping up, and the next thing you know, the whole place smelled of rotten cabbage—the unforgettable odor of phosphines reacting with the air. Nobody wants to repeat that.
It’s not just about avoiding stink. Trihexylphosphine will react with oxygen, producing dangerous byproducts and risking fire. For this reason, laboratories stick to a few tried-and-true rules. Glass bottles with airtight seals become everyday tools. An inert atmosphere, usually provided by a glovebox filled with argon or nitrogen, beats the risk. Stores forget about the open air—every transfer, every weighing, it happens away from that.
Many research guides point to cool, dry storage. I’d vouch for that based on experience. High temperatures might not set off a fire outright, but unwanted reactions speed up—leading not only to waste but to possible accidents. Store it in an explosion-proof cabinet rated for flammable materials, far from heat sources, and definitely away from oxidizers like peroxides or nitric acid. I’ve seen storage mistakes cost more in clean-up and replacement than the proper storage gear ever would.
Anyone who’s ever splashed a strong-smelling chemical across a lab coat understands the value of diligent safety habits. For trihexylphosphine, storing it out of the open—never on a regular shelf—does more than prevent chemical waste. It keeps your skin and lungs safe, too. Spill kits, fire extinguishers, and, most importantly, well-practiced emergency procedures should be in place. I remember one lab that held monthly drills for this kind of incident; nobody enjoyed it, but the rare day something went wrong, you saw how confidence pays off.
Some researchers see these protocols as a burden, but I see them as routine that lets you sleep at night. Material safety data sheets back it all up—store in a sealed container, avoid exposure to air and light, label everything clearly, and keep it away from incompatible substances. Institutions that rigorously train staff and students in these practices wind up with fewer accidents and less down time. The data is clear: more instruction, fewer injuries.
Still, mistakes happen. Training sometimes gets rushed, or equipment isn’t kept in perfect shape. Regular checks and refreshers make a big difference. Labs should encourage open discussion about mistakes so the whole team learns. In my experience, a culture of honesty and attention, rather than finger-pointing, leads to better safety.
Even if your work never touches trihexylphosphine, the core lesson applies. Handle dangerous materials on their terms, with respect, information, and good habits. I’ve run enough experiments to know that a few extra seconds of care can save months of headaches.
Trihexylphosphine isn’t the sort of chemical most people keep under the sink. It shows up more often inside research labs, specialized industries, or chemical syntheses. Anyone who’s ever spilled something nasty while wearing nitrile gloves knows chemicals demand respect. Trihexylphosphine, with its heavy, oily consistency and garlicky odor, will remind anyone in its presence to stay alert.
You find this phosphorus ligand in catalyst systems, where it helps stitch together other molecules in reactions. Most commercial uses flow through specialized hands and don’t wind up in the public space. That's good, because trihexylphosphine brings some baggage.
Accidental exposure to organophosphine compounds like trihexylphosphine can flip a lab day upside down. Even small exposures to the skin or eyes may cause irritation, redness, and sometimes a burning feeling. Inhalation, though less common, can become a genuine emergency if enough vapor builds up inside closed spaces. Acute exposure might mess with your respiratory system. Those strong, almost stinging odors signal danger: take them seriously.
From a chemical stability angle, this stuff reacts quickly with oxidizers and acids. It can catch fire or release harsh gases such as phosphine. Uncontrolled mixing or improper disposal doesn’t just ruin samples or glassware; it can mean real harm for anyone nearby. Emergency showers, eye wash stations, and solid training in handling hazardous spills aren’t just for show.
Documented cases show that phosphine derivatives, if handled poorly, contribute to poisoning incidents. The Centers for Disease Control (CDC) and National Institute for Occupational Safety and Health (NIOSH) identify skin absorption and inhalation as serious exposure routes for similar organophosphine chemicals. Research papers point to the fact that these compounds can affect neurological and liver function if exposure is significant.
Even with gloves and fume hoods, surprises still happen. I remember working on a project where a pipette slip splashed a tiny amount of a similar phosphine on my skin. The itching and burning didn’t last forever, but the lesson did. Cleaning it up involved more care than most folks use making dinner. Specialists keep antidotes and emergency procedures on hand precisely for these moments, especially in industrial and university research settings.
Preparation and training work far better than luck. Anyone using trihexylphosphine should store it in airtight containers, away from acids and oxidizers. Spill kits need materials specific for phosphorus compounds. Standard gloves sometimes fail, so using chemical-resistant gloves—think butyl rubber—helps keep hands out of trouble. Good ventilation, either through fume hoods or local exhaust, stops those sneaky vapors from building up and putting people in harm’s way.
Waste management deserves just as much attention. Dumping leftovers or reaction byproducts into regular trash runs the risk of dangerous chemical fires or toxic gas. Partnering with certified hazardous waste disposal services avoids a lot of headaches for both individual labs and larger organizations.
Manufacturers and research institutions should regularly review their safety data sheets, update training, and keep communication lines open for reporting near-misses or incidents. Open curiosity shouldn’t get stifled, but a careless move with trihexylphosphine can turn one mistake into a scramble for the nearest eyewash station, or worse.
Trihexylphosphine doesn’t show up in daily life like bleach or gasoline, but those who spend their hours in chemistry labs know just how powerful—or dangerous—it can be. This kind of substance has earned a tough reputation for a reason. Out in the world, people may worry about household cleaning sprays, but Trihexylphosphine asks for a whole different level of respect.
I still remember that sharp garlic odor from my earliest days in the lab. It only takes one careless moment to realize a splash could ruin your week—or your lungs. Fumes can hit before your eyes ever see a spill, so relying on your nose or eyesight falls short. Trihexylphosphine’s high reactivity, especially with air and water, pumps up the danger.
Fires and toxic gases sneak up fast. I learned that accidents don’t always look dramatic: just a cracked stopper or damp glove can change everything. Trust in safety isn’t much good if you don’t apply it, so keeping careful becomes a daily routine.
Start with good chemical goggles—better yet, splash-resistant ones that seal tight. Standard safety glasses won’t cut it here. Use heavy-duty gloves built for organophosphines, and double up if you can. Cotton or latex can break down too fast, so nitrile or butyl rubber makes more sense. Dust masks don’t stop fumes—full-face respirators rated for organic vapors take the job seriously.
Some labs keep alcove-style workstations just for volatile phosphines. If you don’t have a designated spot, then always default to a properly functioning fume hood. Strangely, I’ve seen folks open bottles outside the hood for “just one transfer”—and every shortcut risks more than just your shift.
In practice, bottles arrive with sealed caps and sometimes under nitrogen. The aim is to keep out air and water at all costs. Plan every transfer from start to finish before opening the package. Set up gas-tight syringes for measuring and always know where the nearest chemical spill kit sits. Spills involving Trihexylphosphine won’t clean up with a paper towel and a prayer.
Storage stays just as serious. Dry, cool, and dark places work best. Rusty cabinets or loose lids invite leaks. Military-grade secondary containment, like metal bins, gives backup if a bottle tips or cracks. I don’t just stack containers—I check seals and replace questionable packaging on a schedule. That level of care cuts down on surprises.
No matter how prepared you feel, accidents remain possible. Keep antidotes and emergency supplies within reach—medical professionals and poison control always need an up-to-date safety data sheet ready in hand. Eyewash stations and showers should stay clear at all times. I’ve seen drills skipped on busy days; more than once, those missed practices slowed real responses just enough to matter.
Training goes beyond reading a binder. Regular refreshers, hands-on demonstration, and honest discussions about near-misses help everyone remember what’s at stake. You don’t need to work in a chemical plant to value coordinated safety habits—anyone handling serious compounds owes it to themselves and those nearby.
Respect for Trihexylphosphine grows from habit, not fear. Most labs take pride in their track records, but real safety culture shows through quiet routines: labeling every bottle, inspecting gloves before use, running fume hoods before starting reactions. These details never look heroic, though they keep labs running and people healthy. As someone who’s lived with these risks, I can say nothing matters more than staying alert and keeping those routines strong.

| Names | |
| Preferred IUPAC name | Tris(hexyl)phosphane |
| Other names |
Triphenylhexylphosphine
Hexylphosphinotriphenyl |
| Pronunciation | /traɪˌhɛksɪlˈfɒsfiːn/ |
| Identifiers | |
| CAS Number | 28425-87-4 |
| 3D model (JSmol) | `3D model (JSmol)` string for **Trihexylphosphine**: ``` P(C6H13)3 ``` |
| Beilstein Reference | 1719879 |
| ChEBI | CHEBI:33155 |
| ChEMBL | CHEMBL2223635 |
| ChemSpider | 168369 |
| DrugBank | DB12302 |
| ECHA InfoCard | RCRA-1C6B-HC95 |
| EC Number | 244-885-0 |
| Gmelin Reference | 52976 |
| KEGG | C18834 |
| MeSH | D017937 |
| PubChem CID | 12340 |
| RTECS number | TH6825000 |
| UNII | A27K729G9P |
| UN number | UN2810 |
| Properties | |
| Chemical formula | C18H39P |
| Molar mass | 354.64 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | unpleasant |
| Density | 0.824 g/mL at 25 °C |
| Solubility in water | insoluble |
| log P | 4.8 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 28.6 |
| Basicity (pKb) | 6.92 |
| Magnetic susceptibility (χ) | -25.10×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.466 |
| Viscosity | Viscous liquid |
| Dipole moment | 2.19 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 504.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -4182.7 kJ·mol⁻¹ |
| Hazards | |
| GHS labelling | GHS02, GHS06 |
| Pictograms | GHS05,GHS07 |
| Signal word | Danger |
| Hazard statements | H228, H302, H314, H411 |
| Precautionary statements | P210, P222, P231, P235, P302+P335+P334, P308+P313, P370+P378 |
| NFPA 704 (fire diamond) | 1-3-1-W |
| Flash point | > 113 °C |
| Autoignition temperature | 215 °C |
| Lethal dose or concentration | LD50 (oral, rat) 230 mg/kg |
| LD50 (median dose) | LD50 (median dose) of Trihexylphosphine: "815 mg/kg (rat, oral) |
| NIOSH | SY3600000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | glove box |
| Related compounds | |
| Related compounds |
Triethylphosphine
Triphenylphosphine Trimethylphosphine |