
Phosphines have carved their place in modern chemistry for over a century. Triisobutylphosphine, in particular, took its shape along the rising interest in organophosphorus ligands during the mid-1900s when chemists dug into practical solutions for more rugged metal-catalyzed reactions. Back in those early decades, scientists struggled with air-sensitive and sometimes pyrophoric compounds, but persistence pushed the field forward. By the time the late 1970s rolled around, growing demand for more flexible and less volatile phosphine ligands led to experimentation with branched alkyl groups—like those in triisobutylphosphine—creating new tools for synthesis. The chemical’s journey reflects a story of resourcefulness rather than clean lines of discovery: trial, error, and repeat until improvements in synthesis, handling, and safety produced the version familiar to labs today.
Triisobutylphosphine shows up as a clear to pale yellow liquid, giving off a sharp, almost fishy odor typical for organophosphines. Labs often see this chemical in custom glass or stainless-steel containers, sometimes under a nitrogen blanket, which keeps light and air at bay. Though not a household name like triphenylphosphine, it has a reputation among catalysis folks and researchers aiming for more electron-rich, sterically demanding ligands. Most reputable chemical suppliers, whether in the US, Germany, or China, offer triisobutylphosphine with purities above 97%, which caters to research, pilot plants, and scale-up process investigations.
Triisobutylphosphine carries a molecular formula of C12H27P and a molar mass around 202.32 g/mol. It boils in the range of 160–170°C under reduced pressure, which already hints at its relative volatility compared to heavier phosphines. The density hovers near 0.81–0.83 g/cm³, lighter than water, and it floats in the organic solvent category: it dissolves well in typical aprotic media such as toluene, hexanes, and ether, but resists mixing with water. The chemical’s strong, characteristic smell comes from its high vapor pressure. That same volatility presents both handling challenges and opportunities, especially for those who need quick ligand exchange in catalyst systems.
Good practice demands clear, comprehensive labeling for triisobutylphosphine. Anyone handling it should find the CAS number 1070-41-7, along with hazard pictograms, signal words like “Danger,” and hazard statements such as “flammable liquid and vapor” and “harmful if inhaled.” The labeling must highlight water-reactivity and the need for inert conditions. Shipping paperwork should flag it as a potentially dangerous good under transport regulations. Recent trends favor bottles in amber glass with septum tops to allow needle withdrawal in glove boxes or on Schlenk lines, and clear batch purity, water content, and chemical assay information on each package. It comes down to keeping people informed, not just crossing off compliance boxes.
Industrial-scale production typically starts with phosphorus trichloride reacting with isobutyl magnesium chloride, forming triisobutylphosphine in a two-step process. The Grignard approach helps chemists swap out chloride for alkyl groups, and the procedure calls for controlled low temperatures and slow addition to manage exotherms and off-gassing. In research settings, smaller runs still rely on glovebox or Schlenk-line techniques to exclude air and moisture. Final purification steps involve vacuum distillation, careful drying, and assessment for peroxides, since even a tiny impurity of those can spoil a batch and introduce new hazards. The chemical’s trickiest part is often the clean-up, rather than the reaction itself: you feel the weight of frustration when an impurity or misplaced moisture ruins a flask’s whole yield.
Triisobutylphosphine takes on a central role as a ligand for transition metal catalysis. Its bulky, electron-rich nature makes it a favorite for some palladium and nickel catalyzed couplings—like Suzuki or Heck reactions—because it stabilizes low-coordinate metal centers and encourages higher activity. Its willingness to react with oxidizers slices open the possibility for oxidation to phosphine oxides, but those same characteristics make it a risk if left exposed to air. Researchers sometimes tweak it—swapping isobutyl groups for sterically bigger or smaller chains—so they can tune coordination environments around metals for specific syntheses. The compound shows little patience for acidic or strong oxidizing conditions; it either decomposes or reacts violently, so real-world labs keep things cool, dry, and away from open flames or spark sources.
Working with triisobutylphosphine means hearing a small handful of names tossed around. The IUPAC name reads as “triisobutylphosphane,” but most folks in the field go with “TIBP.” Some catalogs might mark it as “phosphine, triisobutyl-,” “tris(2-methylpropyl)phosphine,” or its registry number for clarity. No matter how it shows up on the label, lab regulars recognize it right away by its smell and reputation for handling challenges. The range of synonyms helps make sure cross-checks don’t miss a bottle tucked away under a less-familiar name—which anyone running a crowded solvent store will recognize as a real-world concern.
Triisobutylphosphine raises a flag for both chemical safety and lab culture. The compound demands strict inert handling since air and moisture degrade it and create noxious byproducts. Fume hoods, Schlenk lines, or gloveboxes become an everyday reality. You want good gloves, splash goggles, and a sense of caution drilled in by the first whiff you catch through a loose glove. Storage uses tightly sealed bottles, a cool dark environment, and plenty of labeling. Its flammability and ability to catch fire from static discharge mark it out as more than a nuisance. Spills and leaks go straight to absorbent pads and sealable waste cans. Emergency procedures don’t stay locked away in a binder; teams run through drills because, at the wrong moment, a careless move puts both person and project in danger.
You find triisobutylphosphine showing up in many catalytic cross-coupling reactions, especially those in the pharmaceutical and fine chemicals industries. Its steric bulk and electron-donating power help stabilize nickel and palladium complexes, which matters when tricky substrates call for strong, stable ligands. Some manufacturers use it for processes up to the pilot scale, but its high reactivity keeps it out of most consumer goods. Advanced material synthesis and electronics research dip into this phosphine for specific organometallic preparations—molecular wires, semiconductor precursors, or custom polymers. The number of fields using TIBP doesn’t match broader chemicals, but wherever top-tier catalyst performance or specialized coatings show up, you sometimes find its fingerprint in the lab logbook.
Over the past decade, chemists have pressed triisobutylphosphine into service for cross-coupling reactions and asymmetric catalysis. A steady stream of journal articles and patents trace out efforts to fine-tune its structure for even higher selectivity, turnover numbers, and reduced byproduct formation. Pharmaceutical labs experiment with TIBP derivatives for late-stage functionalization, and small startups gamble on it for new catalytic cycles in green chemistry. The technical literature points toward optimization—methods for faster, cleaner, and less hazardous reactions. Some projects chase lower toxicity, either by engineering less volatile phosphine analogs or by switching to bench-stable complexes that only release TIBP under reaction conditions. Researchers understand that practical value hinges on real workability, not just theoretical advantages printed in tables.
The health effects of triisobutylphosphine matter when balancing utility and safety. Phosphines as a group can cause headaches, nausea, and respiratory irritation, especially after inhalation. Animal studies with related compounds reveal strong pulmonary response to inhaled vapors, and repeated exposure risks chronic symptoms. Laboratory records describe acute effects from accidental splashes or poorly ventilated spaces, and fire department reports back up the need for caution. The compound’s tendency to form dangerous oxides if handled carelessly makes it a constant concern on safety audits. The industry leans on engineering controls, not just personal protective equipment, and builds redundancy into fume extraction and monitoring systems for good reason. The toxicology data stay in front of chemists, not just regulatory agencies: in practice, the best safety protocol is a checklist and a clear head, not just an MSDS on file.
Triisobutylphosphine’s outlook rides on the march of catalysis and material sciences. As industries push for greener, more efficient process chemistry, calls for ligands that drive lower-waste, higher-yield reactions won’t let up. Researchers eye TIBP’s structure, looking for ways to swap out hydrogen and carbons for better solubility profiles or stronger electronic push. A few teams experiment with tethered or immobilized variants to make handling safer and recovery easier. Sustainability-minded labs hunt for alternatives with lower human and environmental toxicity but recognize that real breakthrough requires both chemical smarts and hard-won experience at the bench. The compound’s future comes down to practical trade-offs: cost, safety, and raw performance. Whoever manages to tip the balance in favor of safer, more reliable variations will shape the next decade of phosphorus chemistry.
In the world of specialty chemicals, triisobutylphosphine holds a specific place that most people outside labs or factories rarely notice. This colorless liquid, composed of three isobutyl groups attached to a phosphine backbone, tends to show up in places where precision counts and small tweaks in chemistry shift whole industrial processes. It’s not a household staple, but for those working hands-on with catalysts and fine chemicals, triisobutylphosphine becomes something like a hidden tool — key for unlocking reactions that wouldn’t happen otherwise.
Catalysts drive industries — from plastics to pharmaceuticals. Manufacturers look for ways to make reactions go faster, cleaner, and with fewer byproducts. Triisobutylphosphine often pops up as a ligand for transition metal catalysts, especially in areas like hydroformylation or certain polymerizations. Its bulky structure helps sculpt the reaction environment so that the metal center interacts just right with other molecules. This leads to better selectivity, meaning less waste and more of the product you’re actually aiming for. Real progress in sustainable chemistry means finding tools like this, ones that shape processes and reduce environmental headaches at the source.
Drug development has always demanded reagents that allow chemists to build complex molecules step by step. Triisobutylphosphine plays a role in carrying out selective transformations. A chemist might use it to help create tricky carbon-phosphorus bonds or facilitate coupling reactions where standard phosphines fall short. This adaptability turns into real-world impact every time a new drug molecule gets made more cleanly or at greater scale. Skipping the need for massive purification means faster paths from bench to patient, and less chemical waste left over.
The tech world continues looking for new materials to fuel faster chips and brighter screens. In this quest, triisobutylphosphine sometimes acts as a precursor for metal-phosphide nanocrystals. Materials like indium phosphide benefit from phosphine ligands that control the size and surface properties of each nanocrystal. This leads to better efficiency in devices such as LEDs or solar cells, where even small chemical shifts affect performance. Progress here depends on understanding the craft of chemical control, not just flashy breakthroughs.
Working with triisobutylphosphine brings safety lessons you don’t forget. Phosphines often carry reputations for strong odors and toxicity. Handling them means putting on gloves, working under a fume hood, and double-checking containers are sealed up tight. Mishandling can lead to headaches, respiratory problems, or worse. Responsible use always starts with respecting the hazards — a lesson learned from every spilled drop or nose-wrinkling whiff in the lab.
As green chemistry rises in importance, chemicals like triisobutylphosphine offer a path forward by making existing reactions cleaner or unlocking new pathways altogether. Imagine new bio-based plastics or drugs powered by catalysts tuned with designer phosphines. More research may improve safety profiles or reduce cost, making these specialty reagents available outside the world’s biggest labs. Real advancement comes from both the chemistry and the care taken in its use — not just what happens in the beaker, but how those choices reach industries and, finally, everyday life.
Triisobutylphosphine stands out for its distinct structure: a phosphine where a phosphorus atom bonds with three isobutyl groups. The chemical formula is P(C4H9)3, sometimes written as C12H27P. Each isobutyl group, with the arrangement (CH3)2CHCH2–, attaches to the phosphorus. The result is a bulky, hydrophobic ligand that reacts differently from more basic phosphines.
Focusing on my own time in a chemical lab, the minute you use triisobutylphosphine as a ligand, the whole mood of the reaction can change. Its size blocks unwanted molecules from crowding around a catalyst, giving chemistry teams the chance to drive more selective reactions. That behavior rarely appears with basic, smaller phosphines like triphenylphosphine. The way those isobutyl arms wrap around phosphorus steers the course of reactions—from pharmaceutical syntheses to materials development.
Triisobutylphosphine belongs to a less forgiving group of compounds. It gives off a strong garlic-like odor, and sniffing it too long means asking for trouble—eye and respiratory irritation can follow. Standard lab gloves and goggles aren’t just “suggested”—they absolutely keep skin and eyes safe from burns and allergies caused by organophosphines. In my experience, the chemical’s volatility grabs attention quickly. One spill can have everyone scrambling because organophosphines sometimes catch fire without warning.
Real world use of triisobutylphosphine pops up constantly in industrial chemistry. Oil refineries and specialty plastics both rely on phosphine ligands to coax raw materials into something useful. The bulk and hydrophobic nature of those isobutyl groups change the way metals catalyze certain processes, like hydroformylation, which turns alkenes into aldehydes. In the hands of a skilled chemist, the unique shape blocks side reactions and doubles yields. I’ve witnessed a production line improve dramatically once lab teams switched to this ligand—waste dropped, and the process grew cleaner and faster.
Triisobutylphosphine doesn’t just disappear once a reaction finishes. The phosphorus atom tends to linger in the waste stream. That means extra steps for treatment, so it won’t harm water supplies or soil. Many smaller facilities struggle to afford advanced filtration and incineration, so thoughtful regulation and affordable technology must fill that gap. Reacting the waste with bleach or hydrogen peroxide usually neutralizes the compound, but both chemicals need careful handling too.
Better storage options make a real difference. Sealed containers with minimal air leave less chance for accidents. Automatic ventilation and leak detectors support safety far more than relying on smell. Training lab workers—especially new hires—cuts down on mistakes. Digital tracking of chemical inventories and incident logs help managers spot risky patterns long before they become emergencies. Sharing real accident stories, not just guidelines, brings the lessons home.
Openness fosters trust with the public and regulators. Laboratories that publish safety records or open their doors for local school tours show their care for people and the planet. Letting researchers share failures along with successes means others can learn without repeating the same missteps. Triisobutylphosphine carries risk and reward in equal measure—but with honesty, solid training, and attention to disposal, its benefits win out.
Triisobutylphosphine is a specialty chemical used in laboratories and select industrial processes. Most folks outside a chemistry classroom probably never run into it, but that doesn’t mean it’s not worth talking about. I remember the smell in undergrad labs when we’d handle any organic phosphines—it would linger on your lab coat long after you tied the waste bag. This stuff is known for its strong, unpleasant odor, alerting even the most careless chemist that precautions are key.
Anyone with a background in chemistry hears “phosphine” and thinks “dangerous.” Not just because of the flammability but also the toxicity. According to data from Sigma-Aldrich and the European Chemicals Agency (ECHA), triisobutylphosphine acts a lot like other organophosphorus compounds. It can catch fire easily and produce toxic fumes if burned. Contact with eyes or skin leads to burns and irritation. Inhalation or ingestion could cause far more serious trouble—affecting the lungs, central nervous system, and even the heart.
What’s tricky about triisobutylphosphine isn’t just the acute effects. Many organophosphorus chemicals work behind the scenes by messing with nerve signals. So exposure, even in small doses repeated over time, carries risk. Once in a while I read studies documenting technician injuries or lab mishaps, and the story repeats: improper handling, missing gloves, or a spill during transfer. This isn’t just a problem for lab workers—safety slips impact supply chains, waste handlers, and anyone sharing a workspace.
One time a friend left a bottle of an organophosphine uncapped during a long lunch. By the time he returned, every bench and fume hood stank of garlic, and lab management shut down operations for hours. That safety scare didn’t make headlines, but it summed up the challenge. These chemicals don’t forgive errors, and odors act as early alarms. The U.S. Occupational Safety and Health Administration classifies chemicals like triisobutylphosphine as hazardous, calling for proper ventilation, eye protection, and full coverage clothing. Without that, not only acute burns but long-term effects on breathing and nerve health could follow.
Current research stresses double containment: bottles inside sealed containers, work within a fume hood, thorough cleanup with spill kits. Labs adopting rigorous hazard training report far fewer incidents. Chemical manufacturers sometimes add odorants specifically to warn users, though triisobutylphosphine hardly needs help in that department. Companies have taken to labeling, providing Safety Data Sheets (SDS) online and in physical format. Anyone handling or disposing of this compound should review the SDS before even unsealing a container.
Simple changes matter. A culture where colleagues remind each other about gloves and goggles changes outcomes. Digital recordkeeping tracks usage and storage, highlighting bottlenecks that could cause accidental exposure. Waste handling guidelines, including sealed disposal bins and scheduled pickups, prevent slow leaks and accidental mixing. Many institutions now run chemical safety drills, modeling responses to spills or exposures. Safety doesn’t take heroic gestures—just a steady, careful approach each day.
Triisobutylphosphine’s risks shouldn’t be brushed aside or blown out of proportion. People working with it need up-to-date training, accessible safety gear, and proper waste disposal routes. Companies must commit to transparency and clear labeling. None of this feels glamorous, but these habits protect not only staff but also future students, neighbors, emergency responders, and anyone else who might come into contact with these chemicals.
Triisobutylphosphine stands out for both its usefulness in organic synthesis and its tricky behavior. Anyone who’s worked around organophosphines knows you can’t just leave this stuff lying around. I’ve seen what goes wrong when people get lax about chemical storage. Not everyone realizes how quickly a small oversight snowballs—failing seals, weird smells, fire hazards. Nobody wants to scramble for a response plan in the middle of a spill.
Triisobutylphosphine reacts with oxygen and moisture in the air. These reactions don’t just ruin the chemical; they can also create flammable or toxic byproducts. In the lab, it’s pretty standard to find this compound packed under nitrogen, usually in a well-sealed bottle. That isn’t just a fussy detail—exposure really does wreck its purity, and worse, raises the fire risk.
Everyone storing this material should lock down a few practices. I’ve learned never to rely on memory or good luck. The bottle belongs in a cool, dry place. Direct sunlight is a bad idea. The shelf near the door always gets the most temperature swings—that corner won’t cut it.
A glove box filled with inert gas works well for larger amounts or if people dip into the bottle often. For smaller quantities, a tightly capped container flushed with nitrogen or argon holds up pretty well. Forget putting it in a fridge that also stores lunch (that story never ends well); use a clearly labeled explosion-proof refrigerator.
I've seen people underestimate just how fast these reagents react with air. Even a quick pour or careless transfer introduces enough air to spoil things. Double-check those seals. A little grease on the glass threads helps keep lids tight. Using septum caps with a needle lets you draw what you need without popping the bottle open every time.
Gloves and goggles protect against splashes, but the real danger comes if vapors escape. Good ventilation matters as much as any gloves do. Never skip the fume hood. A minor leak quickly becomes an inhalation hazard.
Spill kits belong close by. Training drills sound boring until someone actually knocks over a bottle. Everyone in the lab should know the plan for leaks, fires, or accidental exposure. The moment panic sets in, a written checklist on the wall saves more than just chemicals.
Handwritten labels fade; print new ones regularly. Include name, date, and hazards. An inventory log helps spot expired or contaminated material before it causes grief.
Some companies use automated systems to monitor temperature and humidity. Alarms catch problems right away, preventing bigger headaches. For smaller operations, just combining a regular check-in with thorough training keeps things running smoothly.
From experience, the biggest difference comes from valuing careful habits and honest conversations. Nobody remembers the day everything worked perfectly. The lessons we all recall come from mistakes, and learning fast keeps the next day safe and productive.
Triisobutylphosphine comes from a family of organophosphorus compounds that are essential for many specialized reactions—think of catalytic processes common in the fine chemicals and pharmaceutical sectors. In my own lab work, I’ve seen how the slightest impurity can throw off a clean reaction, wasting valuable time and resources. For anyone depending on reproducible science, purity transforms from an abstract measure into a real-world obstacle or advantage.
Most commercial suppliers, especially those recognized across international markets, provide triisobutylphosphine with a minimum purity advertised between 95% and 98%. Some specialty grades, meant for highly sensitive research, push purity over 99%. These numbers aren’t just there for show; they represent quality control steps, distillation procedures, and strict handling to avoid contamination by water, oxygen, and organic byproducts.
From what I’ve seen during scale-up processes, even a few tenths of a percent of contamination makes a difference. Unwanted byproducts slip into reactions, affecting both yield and safety. A catalytic reaction, for instance, stops behaving consistently when phosphate or sulfur traces tag along. In our research group, we chased down a mysterious drop in catalyst performance, only to trace it back to a batch of triisobutylphosphine that fell short on purity. Even solvents and containers play a role, turning the whole supply chain into a point of concern.
Laboratories relying on triisobutylphosphine can’t afford to take supplier data at face value. Reputable suppliers issue certificates of analysis, confirming batch-specific values for purity and prominent impurities. Having checked these certificates myself, I know sharp eyes are needed to identify if any “other alkylphosphines” or oxidized phosphine limits creep near the upper allowed levels. Regular in-house analysis by NMR and gas chromatography confirms supplier data and flags any inconsistencies before a whole lot of time or money disappears.
Even if a bottle leaves the manufacturer at 99% purity, exposure to air and moisture chips away at that number in a heartbeat. Triisobutylphosphine reacts quickly with oxygen, so handling has to happen under inert atmosphere, such as nitrogen or argon. I’ve ruined a bottle myself, forgetting to replace the cap or fumbling with a glovebox door. Proper storage isn’t a suggestion; it’s a necessity for anyone wanting reliable results.
Some projects in industry or research use bulk grades where 95% purity fits the budget or technical requirements. For total synthesis or catalyst testing, 98% or higher keeps things on track. Working too close to the minimum causes more troubleshooting than savings. It pays to source from suppliers that demonstrate robust testing and a quick turnaround on technical support. Without that partnership, projects risk delays and unexpected costs tied to off-spec material.
The responsibility for purity doesn’t end with the supplier. Consistent monitoring, careful record-keeping, and smart handling protect both results and reputations. In my own career, I learned early that one contaminated batch means skepticism for every data point that follows. By keeping procedures tight and making sure the bottle matches the purity expected, the chemistry stands on solid ground—and so do any conclusions drawn from it.
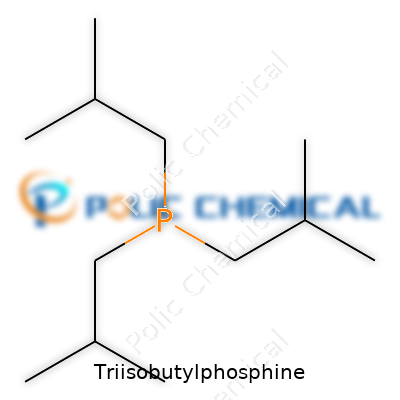
| Names | |
| Preferred IUPAC name | Tri(propan-2-yl)methylphosphane |
| Other names |
TRIISOBUTYL PHOSPHINE
Triisobutylphosphane |
| Pronunciation | /traɪˌaɪsəˌbjuːtɪlˈfɒsfiːn/ |
| Identifiers | |
| CAS Number | 998-40-3 |
| Beilstein Reference | 1711183 |
| ChEBI | CHEBI:33323 |
| ChEMBL | CHEMBL107215 |
| ChemSpider | 72832 |
| DrugBank | DB22294 |
| ECHA InfoCard | 03d8e4e9-26b2-44aa-8121-fbb8d9fda2b6 |
| EC Number | 245-543-3 |
| Gmelin Reference | 6045 |
| KEGG | C18509 |
| MeSH | D017903 |
| PubChem CID | 123178 |
| RTECS number | UF9658000 |
| UNII | HZ1FQ0GW2P |
| UN number | UN3339 |
| Properties | |
| Chemical formula | C12H27P |
| Molar mass | 302.43 g/mol |
| Appearance | Colorless liquid |
| Odor | Unpleasant |
| Density | 0.812 g/mL at 25 °C (lit.) |
| Solubility in water | insoluble |
| log P | 2.8 |
| Vapor pressure | <0.1 hPa (20 °C) |
| Acidity (pKa) | 28.0 |
| Basicity (pKb) | 7.7 |
| Magnetic susceptibility (χ) | -80.50 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.435 |
| Viscosity | 45 cP (20°C) |
| Dipole moment | 3.73 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 354.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -271.7 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H225, H302, H315, H319, H332, H335 |
| Precautionary statements | P210, P261, P280, P305+P351+P338, P312 |
| Flash point | 130 °C |
| Autoignition temperature | 260 °C (500 °F) |
| Lethal dose or concentration | LD50 (oral, rat): 650 mg/kg |
| LD50 (median dose) | LD50 (median dose) of Triisobutylphosphine: 1200 mg/kg (rat, oral) |
| NIOSH | Not listed |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 10 ppm |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Tri-n-butylphosphine
Tri-tert-butylphosphine Tributylamine Triethylphosphine Triphenylphosphine |